
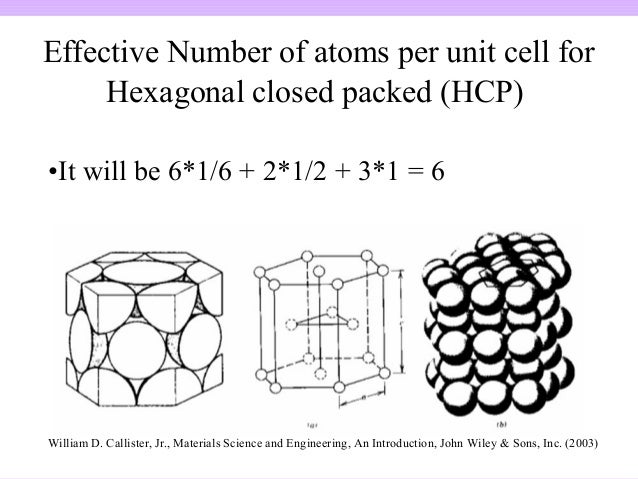
In this video Parisa works through the calculation of the atomic packing factor APF for the body centred cubic BCC crystal structure. The atomic packing factor is defined as the ratio of the volume occupied by the average number of atoms in a unit cell to the volume of the unit cell. Calculate the atomic packing factor of BCC crystal structure. How to calculate the Atomic Packing Factor for face-centered cubic FCC and body-centered cubic BCC crystal structureA.Ītomic Packing Factor for Simple Cubic - no. 5 rows for FCC a 22 r where a is side of the cube and r is atomic radius. Principal of Engineering MaterialsI LecDr.įCC has a higher packing. Number Of atoms per unit cell Fig g 20 7e 1 atom at center 18 th Of 8 corner atoms 2 atoms Stepldentify close packed direction 3 4R of. FCC has a packing factor of 074 and BCC is 068 so FCC has higher packing efficiency Why FCC metals are more ductile than Bcc metals. What is the packing fraction of BCC and FCC.Ĭlick to see the answer. What is the atomic packing factor of BCC. The bcc body-centered cubic crystal structure has an atomic packing factor APF of 068. Of atoms 1 π volume of one atom volume of unit cell cubic a3 when a 2r Filling Factor Atomic Filling Factor for BCC-no of.

Up to 24 cash back FCC has a packing factor of 074 and BCC is 068 so FCC has higher packing efficiency Read More How many atoms per unit cell are there in the HCP crystal.
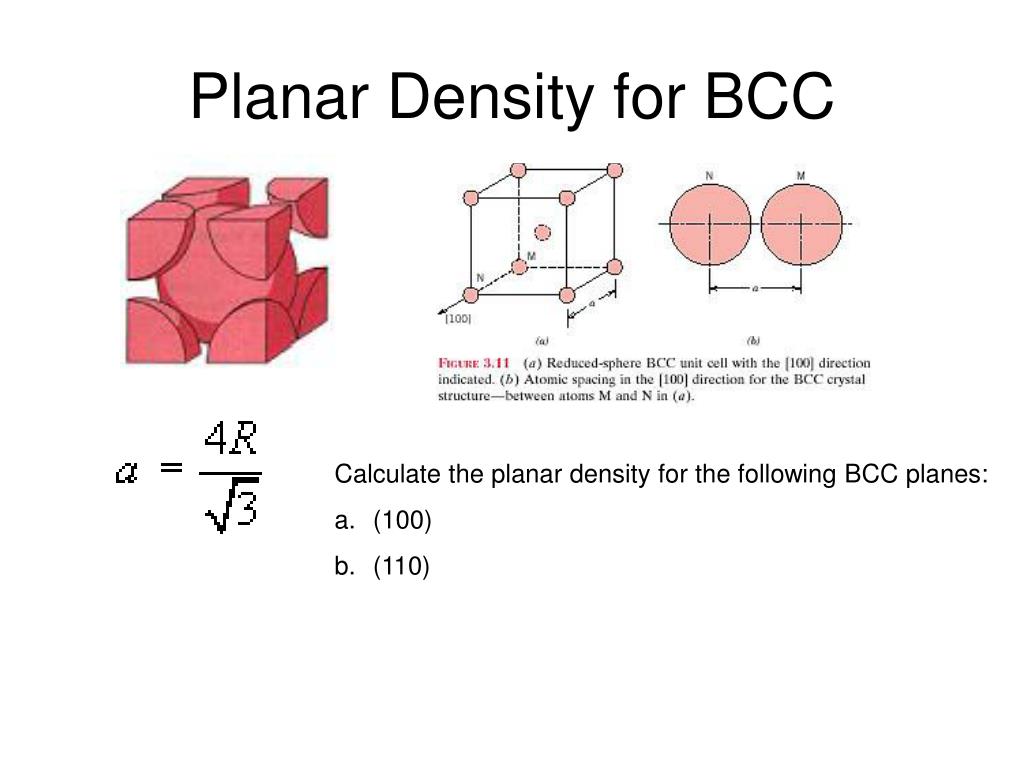
Show that the atomic packing factor for BCC is 068.Ītomic Packing Factor APF for BCC Step 1. Common sphere packings taken on by atomic systems are listed below with their corresponding packing fraction. Planar Packing Fraction Factor For The Body Centred Cubic 111 Plane Fractions Body Packing APF 074. Show that the atomic packing factor for Body-Centered Cubic BCC crystal structure is 068.


 0 kommentar(er)
0 kommentar(er)
